I. Engineering Synthetic Biomembranes
What constitutes a biological cell and can we understand enough about its internal workings to produce an artificial life form? As the first step towards achieving this goal, we are working actively to develop novel methods to create synthetic lipid membranes and compartments. Our interest in this topic reflects our fascination with the process of self-assembly and directed assembly of compartments from colloidal particles and/or macromolecules. Such processes may have played a role in the origins of life.
i. Quantitative studies of giant vesicles
|
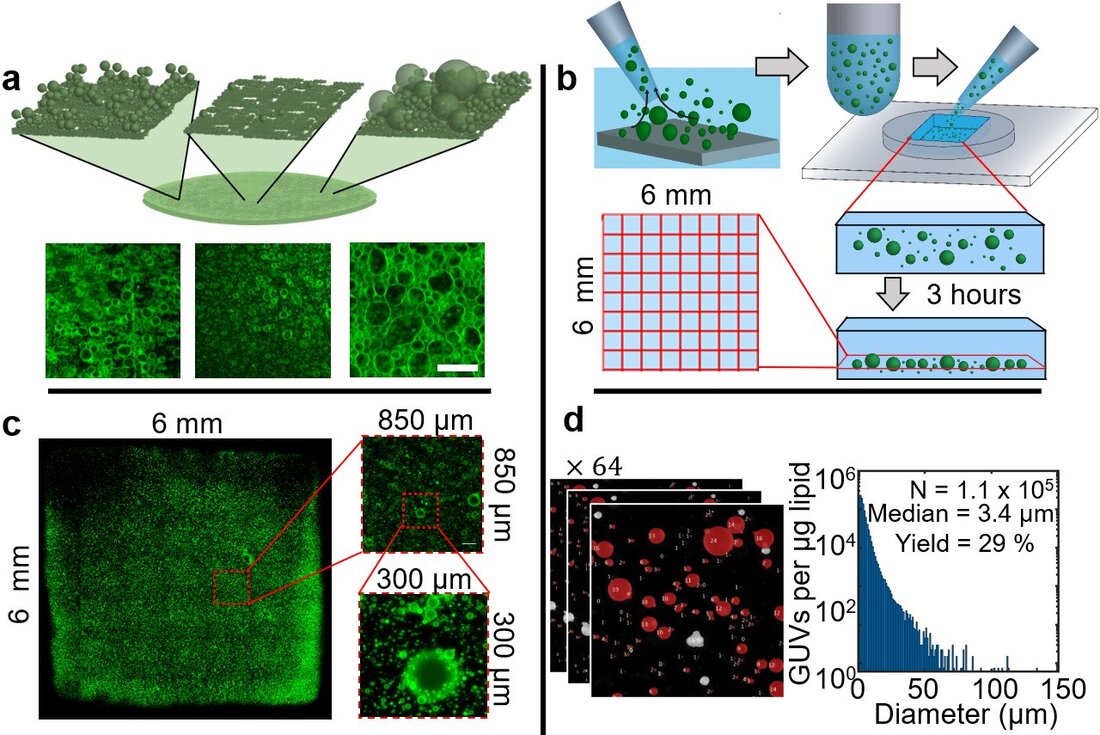
Giant unilamellar vesicles (GUVs) are single-walled closed phospholipid bilayer membranes that resemble minimal biological cells. Our group has developed a quantitative framework using sedimentation, confocal microscopy, and image analysis to measure the molar yield (also known as the percent yield), the absolute yield (also known as the reaction yield), and distribution of sizes of GUVs (Fig. 1). These measurements provide a quantitative metric to understand the effects of physical variables such as the substrate geometry and chemistry, time, temperature, buffer ionic strength, and assisting compounds on the process of assembly. A relatively involved technique was necessary since thin film hydration methods produce GUVs with a wide distribution of diameters that range from 1 micrometer to 150 micrometers. Often, there are other side structures such as multilamellar vesicles, lipid nanotubes, and poorly hydrated lipid debris that must be excluded from the calculation of yield. High resolution imaging and image analysis allows us to identify and exclude these other structures.
|
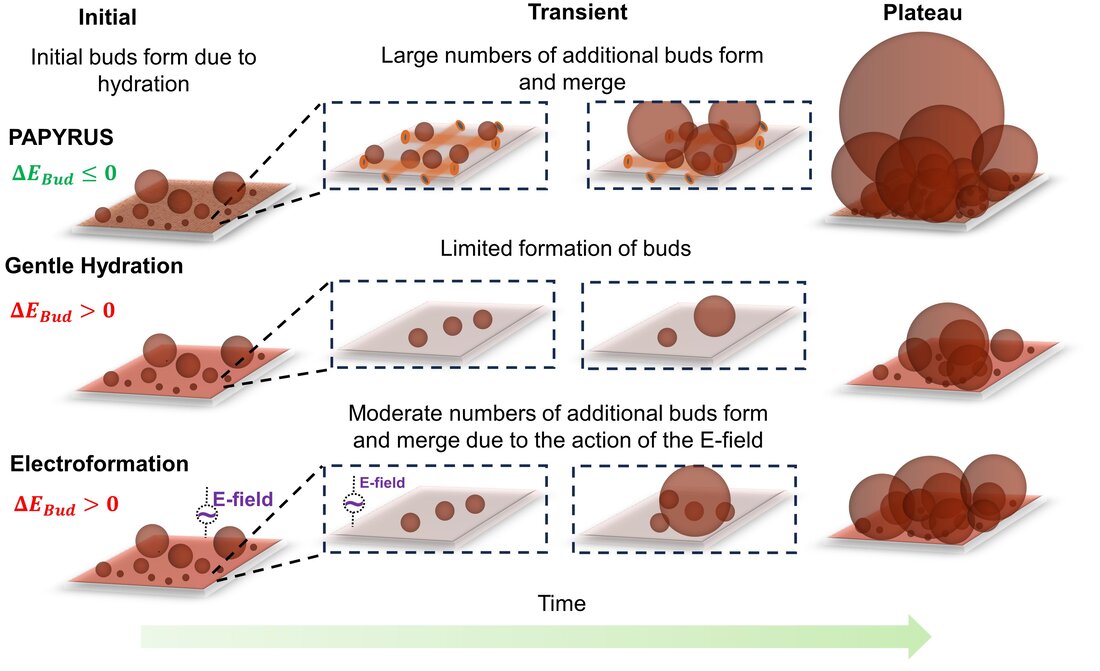
In the first of our papers that prompted the development of this quantitative framework, we compare the relative efficiency of popular thin film hydration methods, electroformation and gentle hydration, with the method we developed, PAPYRUS (Paper-Abetted amPhiphile hYdRation in aqUeous Solutions). The PAPYRUS method uses nanocellulose paper as a substrate to support the thin lipid films. We show that the PAPYRUS method can produce up to double the yield of GUVs compared to electroformation and gentle hydration. We then developed the 'stopped-time' technique to address the question of dynamics of GUV assembly. In the stopped-time technique, we harvest all the GUV buds from the surfaces at specific time points and measure the distribution of diameters and the yields. Plotting these results sequentially in time shows the evolution of these properties in populations of GUVs. Correlating this quantitative data with high resolution time-lapse images of the buds on the lipid films provides insights into the fundamental pathways of assembly.
ii. The budding and merging (BNM) model for the assembly of GUVs
|
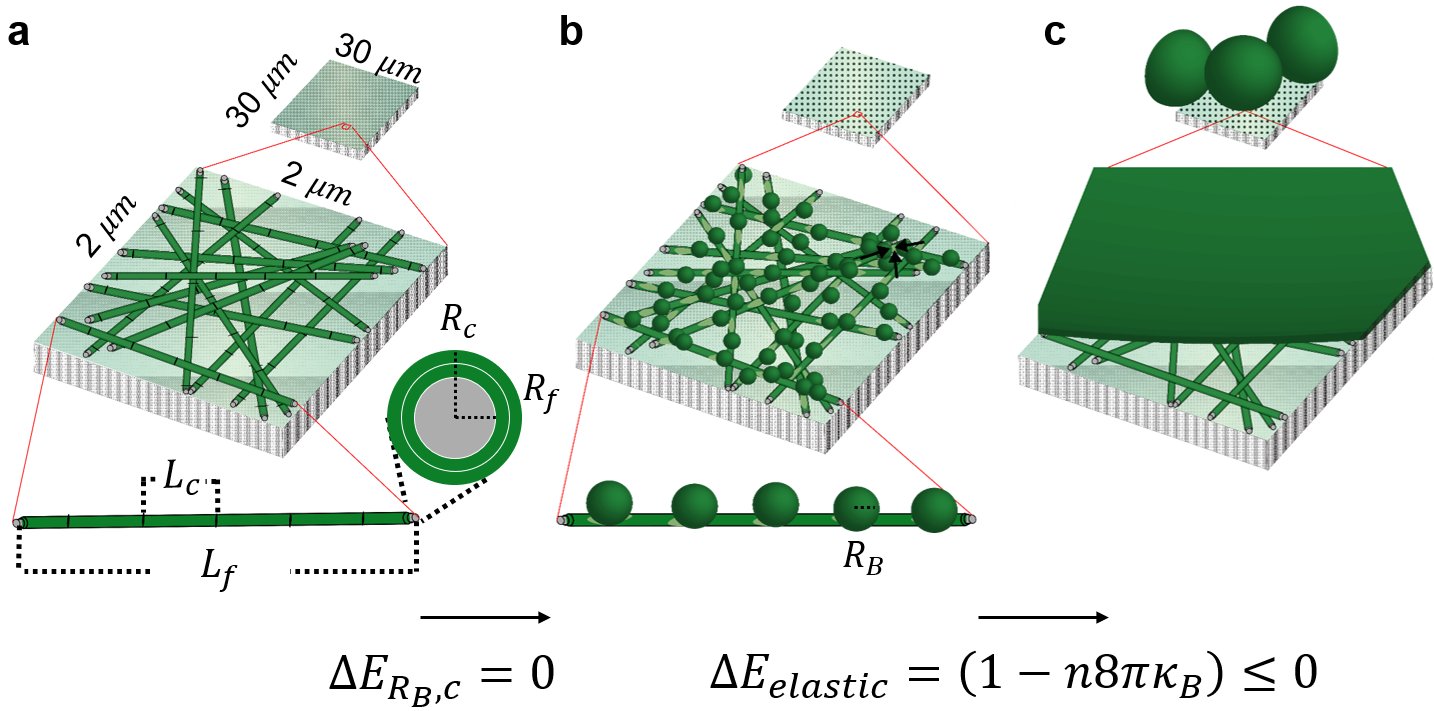
We developed the BNM model to explain the intriguing increase in yield of GUVs when nanopaper was used as a substrate. We noted that a distinct difference between nanopaper and other substrates that have been used before is the geometry of the paper. Nanopaper is composed of enmeshed cylindrical fibers ~ 17 nm in radius and ~ 2000 nm in length. Compared to regular filter paper, nanopaper has a high density of fibers and no micrometer scale pores. The GUVs obtained from nanocellulose paper were large and numerous. Use of regenerated cellulose dialysis membranes, which retains the chemical properties of cellulose, such as hydrophilicity and swellability, but is flat, resulted in yields similar to electroformation. We derived an energy expression that compares the free energy of the lipids configured as a spherical bud versus as a cylindrical or flat bilayer. We showed that the free energy change can be negative for bilayers on nanocylinders while the free energy change is always positive for bilayers on flat surfaces. Once formed, it is energetically favorable for the buds to merge with each other to reduce the total elastic energy of the system. A hypothetical pathway involving both budding and merging provides an energetically favorable path for bilayers on nanoscale cylinders to form GUVs while the path on flat surfaces requires the input of energy (Fig. 2). Dynamically, the GUV yields from PAPYRUS, gentle hydration, and electroformation show a sigmoidal evolution while the size distribution broadens with time. These dynamics were consistent with expectations of the BNM model and the energy landscape of the different methods (Fig. 3).
|
|
iii. PAPYRUS method
PAPYRUS 1.0: Cellulose Filter Paper, Fabrics
We first reported that giant vesicles could be assembled on Whatman 1 filter paper, which introduced the concept of paper-based hydration to the community. We then showed that the formation of giant vesicles was general to fabrics and also general for other amphiphile types such as fatty acids and amphiphilic block copolymers. GUVs were trapped in the micrometer scale pores and there were significant losses of lipid into the paper which necessitated specialized harvesting techniques. Often only small GUVs could be obtained since the large GUVs were trapped in the pores. Understanding the mechanism of how paper promoted the formation of GUVs and the relative yields and sizes of GUVs prompted us to develop GUV quantitation techniques.
PAPYRUS 2.0: Nanocellulose Paper
The current optimized PAPYRUS method uses nanocellulose paper as a substrate. Compared to filter paper or fabrics, nanocellulose paper does not have micrometer scale pores that trap GUVs and is relatively less porous compared to filter paper or fabrics. The lipid can be spread using drop casting similar to conventional methods used for electroformation on indium tin oxide (ITO) coated glass. Nanocellulose paper can be obtained commercially as tracing paper. PAPYRUS 2.0, or simply PAPYRUS, supplants the original report of using Whatman 1 filter paper in terms of ease of use, sizes of GUVs, and yields.
We first reported that giant vesicles could be assembled on Whatman 1 filter paper, which introduced the concept of paper-based hydration to the community. We then showed that the formation of giant vesicles was general to fabrics and also general for other amphiphile types such as fatty acids and amphiphilic block copolymers. GUVs were trapped in the micrometer scale pores and there were significant losses of lipid into the paper which necessitated specialized harvesting techniques. Often only small GUVs could be obtained since the large GUVs were trapped in the pores. Understanding the mechanism of how paper promoted the formation of GUVs and the relative yields and sizes of GUVs prompted us to develop GUV quantitation techniques.
PAPYRUS 2.0: Nanocellulose Paper
The current optimized PAPYRUS method uses nanocellulose paper as a substrate. Compared to filter paper or fabrics, nanocellulose paper does not have micrometer scale pores that trap GUVs and is relatively less porous compared to filter paper or fabrics. The lipid can be spread using drop casting similar to conventional methods used for electroformation on indium tin oxide (ITO) coated glass. Nanocellulose paper can be obtained commercially as tracing paper. PAPYRUS 2.0, or simply PAPYRUS, supplants the original report of using Whatman 1 filter paper in terms of ease of use, sizes of GUVs, and yields.
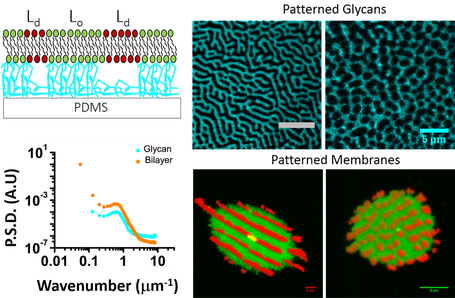
iv. Phase transitions in biological materials
|
The role of extracellular polysaccharides/glycans for the proper functioning of cells is still largely mysterious. Using a multi-pronged approach with tools from surface science, analytical chemistry, and high resolution (temporal and spatial) optics, our group interrogates membrane dynamics and phase behavior close to criticality, as well as far from it. By understanding the interactions between extracellular polysaccharides and lipid membranes, we wish to design structures or materials that interact with membranes, be they in cells or in model systems, to engineer patterns that lead to prescribed functions.
|